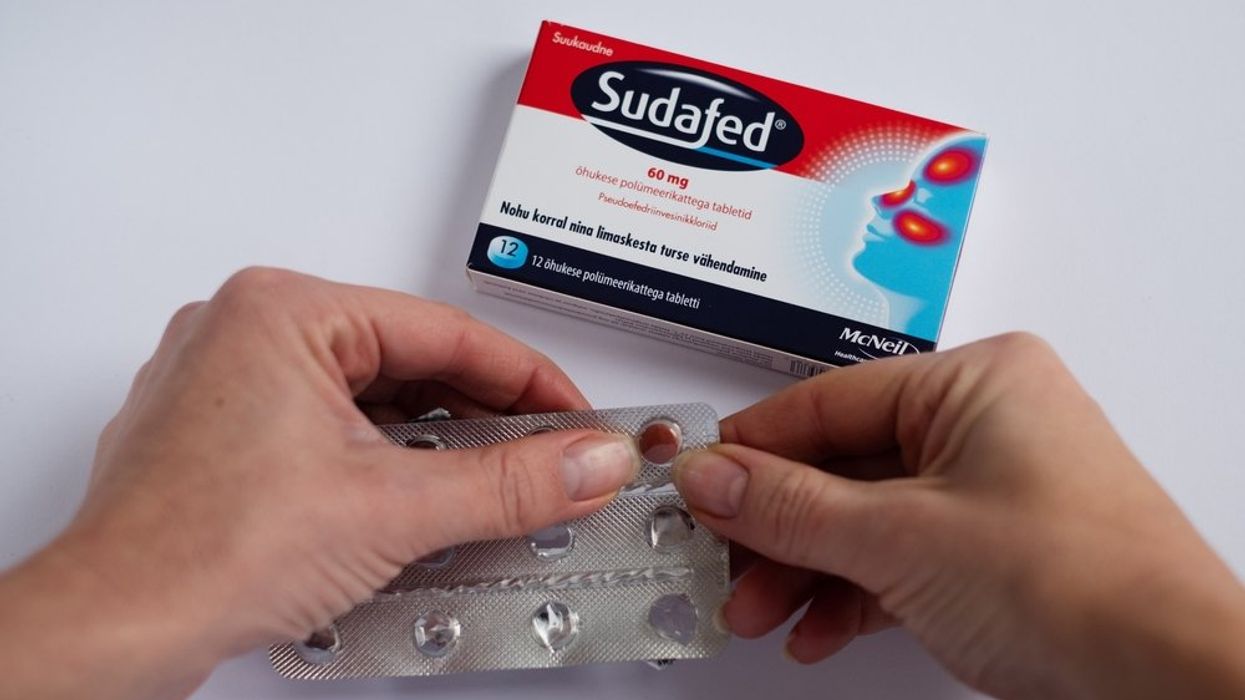
The key ingredient in several over-the-counter cold and allergy medications doesn't actually work, the Food and Drug Administration determined Tuesday.
An advisory panel unanimously determined that phenylephrine, when used in oral form, is ineffective in relieving congestion. Phenylephrine is the most popular oral decongestant in the United States, used in forms of Sudafed, Vicks Nyquil, and Benadryl. It generates nearly $1.8 billion in sales last year, according to FDA data.
Respiratory illnesses surge in Autumn
The drug was advertised as being capable of reducing the swelling of blood vessels in the nose, relieving congestion. FDA officials cited five studies from the past two decades, all of which concluded that the drug was not any more effective than a placebo. In fact, when taken orally, a very small amount of phenylephrine actually reaches the nose.
“In conclusion, we do believe that the original studies were methodologically unsound and do not match today’s standard. By contrast, we believe the new data are credible and do not provide evidence that oral phenylephrine is effective as a nasal decongestant,” said Dr. Peter Starke, the official who led the review.
The FDA will now reconsider the drug's designation as “generally recognized as safe and effective.” Without the designation, any products containing phenylephrine may need to be removed from stores.
Ineffectiveness is not the only concern for the drug, which is known to cause side effects of headaches, insomnia, and anxiety. Higher doses can also lead to an increase in blood pressure.
Still, committee members expressed during the vote Tuesday the desire for the FDA to take action against the drug, primarily because it wastes consumer money when more effective products are available.
Committee member Dr. William Fig, a clinical pharmacologist and investigator at the National Cancer Institute, noted: “It’s amazing the amount of dollars being spent on something that has really no efficacy."